9. Bio-based aviation fuel
9. Bio-based aviation fuel
This book is about advanced fuels based on agricultural biomass resources and their production.
9.2 How are SAFs manufactured?
The basic principles of the most important, already approved production processes for the manufacture of bio-based SAF are explained below. A complete and up-to-date overview of the production processes approved to date by the American Society for Testing and Materials (ASTM) can be found at the ICAO.
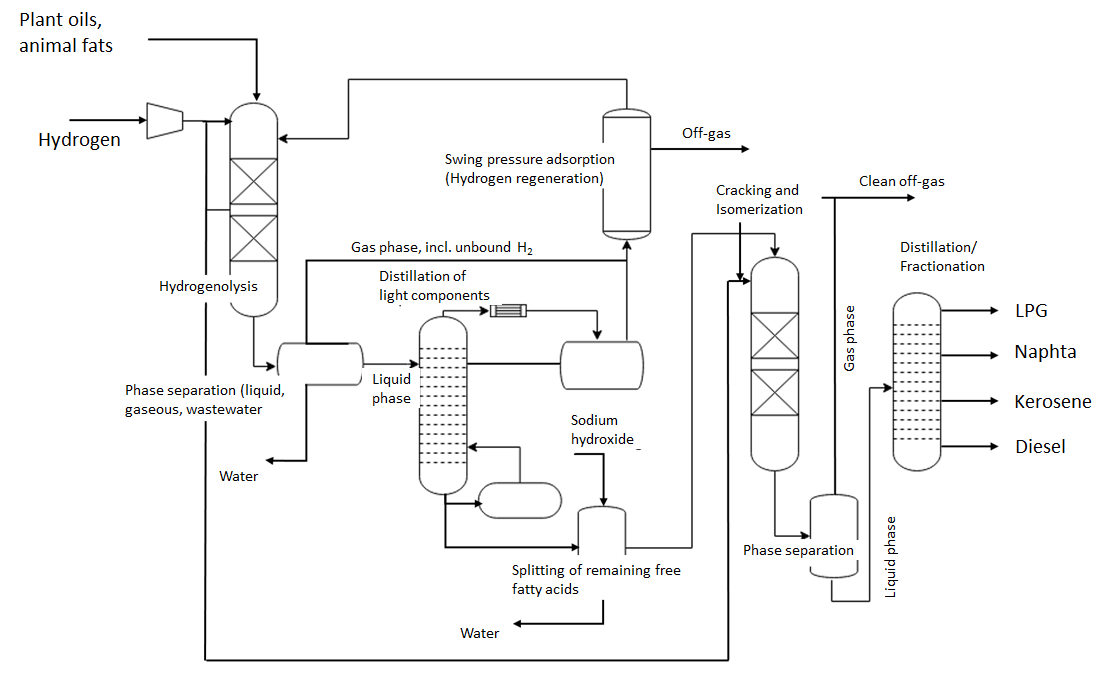
HEFA process
All vegetable oils or animal fats can be used to produce the fuel. However, a process based on algae has also already been approved. On a large scale, used cooking oils (e.g. frying fat) and animal fats from slaughterhouse waste are already being used to produce biodiesel or biokerosene, particularly by the Finnish company Neste.
Process steps
| Process step | Procedure |
| 1 | Cleaning the oil or grease, removing impurities |
| 2 | Esters and double bonds that make up oils and fats must be removed. Hydrogen is used for this - which is why the process step is called hydrogenolysis. Oxygen is also removed from the product as a by-product, as it reacts with the hydrogen to form water. The process requires catalysts made of cobalt or nickel, temperatures of 280-340°C and pressures between 50 and 100 bar. |
| 3 | Combined process of cracking and isomerization, in which the resulting long-chain n-alkanes are converted into shorter, branched molecules and open bonds are saturated with hydrogen. Precious metal catalysts and hydrogen are used for isomerization. The result is a higher quality crude oil that does not become viscous as quickly at low temperatures. |
| 4 | Separation of the product stream into the various alkanes with similar properties. During subsequent distillation and rectification, the remaining liquid products are separated into three fractions - naphtha, kerosene and diesel - based on their different boiling points. Various additives are added to meet the requirements for aviation fuel. |
Flow chart HEFA process
Fischer-Tropsch synthesis from biogas
In order to be able to produce a fuel from municipal waste or forestry and agricultural residues, the solids must first be converted into a gas. This process takes place in biogas plants and was explained in detail in chapter 8 of this learning offer.
The further steps involved in liquefying the gas are briefly explained in the following table.
| Process step | Procedure |
| 1 | Methane reforming is used to produce a synthesis gas containing carbon monoxide (CO) and hydrogen (H2) from the biomethane. Hydrogen to carbon monoxide ratios of 2.1 are most suitable for the next process steps. |
| 2 | In order to achieve the optimum ratios, the syngas must be conditioned. This mainly involves removing CO and adjusting the hydrogen content. Impurities are also removed. The hydrogen content should be at least 50%. The optimum ratio of H2 to CO depends on the atalyzers used in the subsequent Fischer-Tropsch synthesis. |
| 3 | Fischer-Tropsch synthesis is used to produce paraffins, olefins and oxygenated compounds from syngas. Mainly straight-chain n-alkanes, less branched iso-alkanes are produced, which, unlike products made from crude oil, do not contain any sulphur or aromatics. The presence of iron, cobalt or nickel catalysts is required for the reaction. The temperatures and pressures required also depend on the catalyst material used. |
| 4 | Separation by distillation and rectification of the product stream into the fractions kerosene, naphtha, liquid gas and diesel on the basis of the different boiling points. Long-chain waxes are also produced, which have to be cracked again using hydrogen in order to increase the kerosene yield. In order to meet the requirements for aviation fuel, the kerosene fraction is refined with various additives. |
Fischer-Tropsch process steps by Anne Rödl (CC BY), adopted by Jana Schultz
Alcohol-to-jet
In order to produce aviation fuel from alcohol, ethanol or butanol must first be produced from sugar. This is done by means of fermentation (bioethanol production), as explained in detail in chapter 2.1 of the HOOU platform's Future Fuels learning offer.
The actual steps of the alcohol-to-jet process are briefly explained in the following table.
| Process step | Procedure |
| 1 | The dehydration process converts alcohol into alkenes by splitting off water. The process is carried out with the addition of sulphuric acid or phosphoric acid and metal oxide catalysts at 170-200°C. |
| 2 | During the subsequent oligomerization, the alkenes (ethene or butene) combine to form longer-chain molecules. Catalysts are also used here. |
| 3 | The resulting products are separated by distillation and rectification. |
| 4 | The subsequent hydrogenation saturates open double bonds with hydrogen to form alkanes. Nickel, palladium or platinum are used as catalysts. The products are kerosene, gasoline and diesel. |
Synthesized iso-paraffins from hydrogenated fermented sugar (SIP)
The production process is based on the metabolization of sugars to long-chain hydrocarbons by genetically modified yeasts. The C-15 alkenes produced are called farnesenes. Various enzymes and fungi are required for this. Basically, this is also a fermentation process that takes place under aerobic conditions, i.e. under the influence of oxygen. The long-chain farnesenes are separated from the fermenter broth and converted into farnesanes via hydrogenation. Sugar-based synthesized iso-paraffins are currently only produced in small quantities, as the process is still in the demonstration phase. It is questionable whether this type of kerosene will actually be used in large quantities.
It is interesting to note that the anti-malarial agent artemisinin, which occurs naturally in annual mugwort, can be biosynthesized as a by-product of the process. The only manufacturer of SIP at present is therefore concentrating more on bio-based chemical products than on kerosene.
Further information on artemisinin and other active plant substances can be found in the book Pflanzliche Arzneimittel.