- Nutze alle Lernfunktionen, wie Tests, Quizze und Umfragen.
- Schreibe Beiträge und tausche dich in unseren Foren aus.
- In einigen Lernangeboten bestätigen wir dir die Teilnahme.
Nitrogen Chemistry and the Environment
Abschnittsübersicht
-
-
🚀 Welcome to the Course
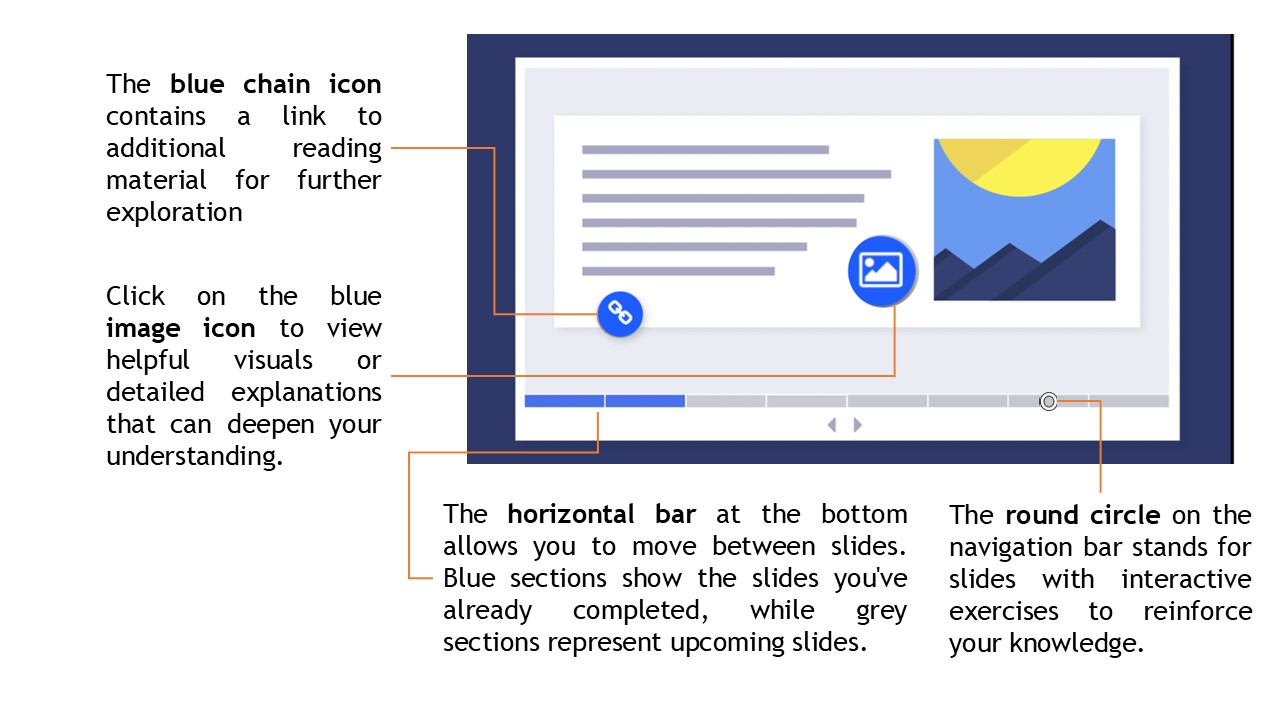
This course is divided into 6 chapters. Throughout the course, you’ll have the opportunity to navigate easily using the interface provided below:
You can skip directly to exercises, where offered, to test your knowledge and reinforce your learning. At the end of each chapter, additional information may be provided to help you explore the topic further.
At the end of the course, you’ll have the chance to share your feedback to help us improve, and generate a participation certificate as a token of your achievement.
Have fun learning, and stay on track!
-
-
-
No Planet B

Does our planet have limits? Johan Rockström, Director of Stockholm Resilience Center, along with 28 planetary scientists tried to answer this very complex question in the report "Planetary Boundaries: Exploring the safe operating space for Humanity".
In this prologue, you will explore the concept of 'planetary boundaries' and its significance for the course.
Below you can see how planetary boundaries were changing over time. Reflect on which change surprises you most? Was there something that you expected to see, and what you never thought of before?
-
-
-
Flow of nutrients interconnects all biotic and abiotic elements in the marine, terrestrial, and atmospheric environments that support life on Earth.
Therefore, any disruptions in a nutrient cycle will have multiple impacts across the biosphere as a whole.
-
-
-
Nitrogen is all around us.
Nitrogen is an element that plays a crucial role in our daily lives, often in ways we might not immediately recognize. For instance, the air we breathe is about 78% nitrogen. What about other properties of this essential element?
An introduction to nitrogen in the environment begins with a paradox: nitrogen — existing in its inert state as N2 — is the most abundant element in the earth’s atmosphere but, in this form, is almost wholly unusable by the vast majority of living organisms. (Braun et al., 2007)
-
If you need to refresh your knowledge of Lewis structures and the octet rule, check out this interactive video!
-
-
-
In the previous chapter, you learned about nitrogen's capacity to exist in a wide range of oxidation states, as well as the strength of the triple bond between nitrogen atoms, which contributes to its remarkable stability and inertness in the atmosphere. Now, let’s take a closer look at the biogeochemical cycle of nitrogen, which is crucial for sustaining life on Earth. This cycle illustrates how nitrogen transitions through various chemical forms and environmental compartments, from the atmosphere to the soil, and ultimately into living organisms.
Reflect on how the processes of nitrogen fixation and biofixation play a crucial role in the nitrogen cycle, and consider how evolutionary adaptations in organisms have enabled them to overcome the challenge of utilizing atmospheric nitrogen.
-
-
-
-
-
In this chapter, you will explore the Haber-Bosch process, an pivotal innovation that revolutionized the production of nitrogen fertilizers. We will examine its scientific foundations and the historical context of its development.
This chapter sets the stage for understanding the legacy of the Haber-Bosch process—its role in feeding the world and its contributions to the nitrogen cascade.
Fritz Haber, who was awarded the Nobel Prize in Chemistry in 1918 for his work on ammonia synthesis, remains a controversial figure. Learn why the award was met with controversy in the video below:
-
-
-
In the previous chapter, we explored how the Haber-Bosch process revolutionized nitrogen fixation and how human activity has introduced significant amounts of reactive nitrogen into the environment. These interventions have disrupted the natural nitrogen cycle, increasing the availability of reactive nitrogen far beyond preindustrial levels.
Now, we turn our focus to the nitrogen cascade—a sequence of environmental impacts triggered as reactive nitrogen moves through air, water, and soil. This chapter examines the pathways of reactive nitrogen, its cascading effects on ecosystems and human health. By understanding the pathways and consequences of reactive nitrogen, we can work toward minimizing its adverse effects on the environment.
„An intact nitrogen cycle is a key systemic requirement for the earth's ecosystems to retain functionality within planetary boundaries” (BMUV, 2017)International and National Initiatives on the Nitrogen Crisis- International Nitrogen Initiative (INI): Focuses on minimizing nitrogen's environmental impacts while optimizing its use in food production.
- Convention on Long-Range Transboundary Air Pollution (CLRTAP): Addresses transboundary nitrogen oxide and ammonia pollution through protocols like the Gothenburg Protocol.
- EU Nitrates Directive: Sets limits on agricultural nitrates to reduce water pollution.
- Global Programme of Action for the Protection of the Marine Environment: Aims to prevent nitrogen-driven eutrophication in coastal regions.
National Initiatives
- Germany’s Integrated Nitrogen Reduction Strategy: Implements cross-sectoral targets for reducing ammonia and nitrogen oxide emissions.
- Danish Nitrogen Action Plan: Combines nitrogen reduction efforts with climate goals, focusing on agriculture and water quality improvement.
- Germany’s Fertilizer Ordinance: Regulates nutrient application in agriculture to reduce nitrate pollution.
-
-
-
Congratulations on completing this course!
Please take a few moments to share your thoughts by filling out the feedback form below.
As a token of your achievement, you can also generate a personalized participation certificate.
Thank you for being part of the Hamburg Open Online University community. We look forward to seeing you in future courses!
-
-
-
This course is published as an Open Educational Resource (OER) and is available in the Edusharing repository for teachers and educators. Link
-