Biofuels from Lignocellulosic Biomass
Fischer-Tropsch Synthesis
The so called synthol process was invented by Franz Fischer and Hans Tropsch in the early 1920s. The process was then continously improved and the influence of process parameters like temperature, pressure, and catalysts where analysed and optimized.
The basic reactions can be described by:
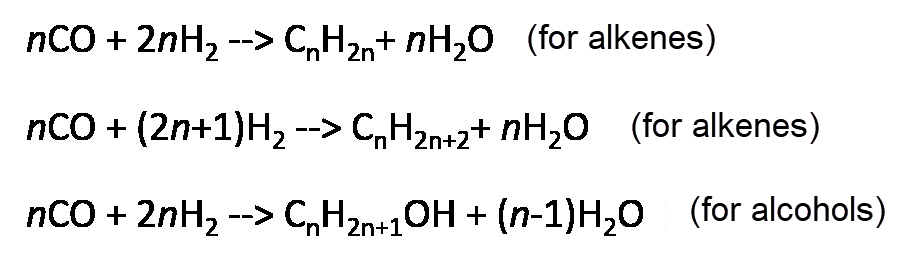
Basic Reactions of Fischer-Tropsch Synthesis_EN by Anne Rödl (CC0)
The exact reaction mechanism is not fully understood until today because of its complexity. During FT synthesis various different reactions take place. See more details of the reaction mechanism in Rauch et al. (2018 “Biokerosene Book”).
The length of the obtained hydrocarbons ranges from C1 (methane) to C20+ (waxes). Mainly unbranched molecules (i.e. n-alkanes) are produced. The chain length distribution in the final product is determined by the chain growth probability (usually between 0.7 and 0.9). Higher values increase the share of waxes (long chained hydrocarbons). The chain growth probability is influenced by temperature, pressure, catalysts, reactor type and H2/CO ratio in the syngas.
The reaction already proceeds at atmospheric pressure. With increasing pressure, the processes run more efficiently.
Catalysts such as iron, cobalt, nickel, or ruthenium are used. The choice of catalyst significantly influences the spectrum of synthesis products. Cobalt, for example, promotes the formation of alkanes. Ruthenium is the most active catalyst for the FT (Fischer-Tropsch) synthesis but is rarely used due to its high cost.
For the FT process, fixed-bed, fluidized-bed, or slurry reactors can be employed. Fixed-bed reactors are often designed as multi-tube reactors (see figure below). The tubes allow for cooling, as the synthesis is a highly exothermic process. The tubes are filled with catalysts. The smaller the catalyst particles, the better the conversion rate. The coolant surrounds the tubes.
The process can be designed to recycle unconverted gases back into the reactor, increasing conversion efficiency (full-conversion mode). However, the process can also be designed without this complex recycling step.
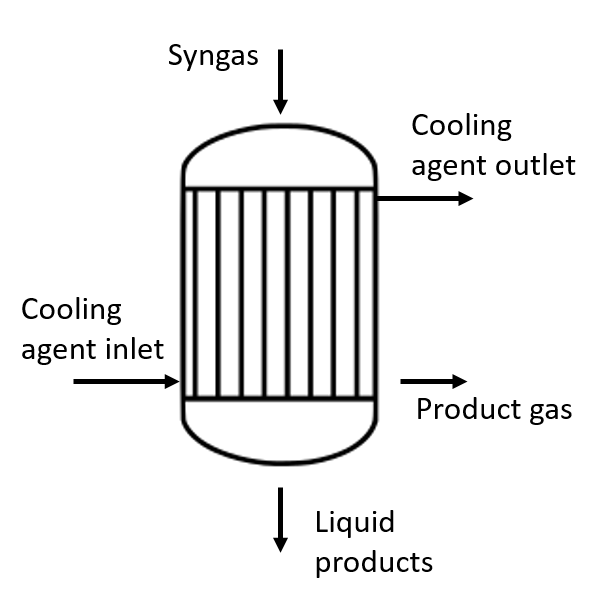
Multi-tubular reactor for FT synthesis von Anne Rödl (CC BY-SA 4.0)
The final products mainly consist of alkanes and are free of sulfur and aromatics. The last step involves the refining and separation of the FT synthesis products, which is done through hydrocracking and distillation or rectification. During hydrocracking, long-chain hydrocarbons are broken down into products with lower boiling points (e.g., naphtha, kerosene, or diesel) by adding hydrogen. Cracking is also facilitated by catalysts (e.g., nickel). At the same time, isomerization occurs, where long-chain hydrocarbons are converted into branched hydrocarbons. In distillation, the desired fractions are separated based on their boiling points.
The exact reaction mechanism remains incompletely understood due to its complexity. Various reactions occur during the FT synthesis. Further details on the reaction mechanism can be found in: