Biodiesel (Oil- and Fat-Based Biofuel)
Biofuels from Iso-Conversion
The Biofuels Iso-Conversion (BIC) process enables the conversion of used fats and oils into biofuels. The process begins with a hydrothermal cleaning step to remove impurities. The first step in the conversion is catalytic hydrothermolysis, where triglycerides and free fatty acids are converted into straight-chain and cycloalkenes in a water atmosphere under supercritical conditions. This process takes only 2 minutes. The feedstock is mixed with water and treated in a reactor at 500-600°C and 200-250 bar. Triglycerides are broken down into intermediates such as organic acids and alkenes, and n-alkanes are converted into cycloalkanes (aromatic compounds). In the next step, the olefins are saturated, and oxygen is removed using hydrogen and non-noble metal catalysts during conventional hydrotreating. Afterward, the products are fractionated in distillation columns, yielding liquefied gas (propane, butane), naphtha, kerosene, and diesel. Currently, only a pilot plant is in operation, but the process shows potential for large-scale application.
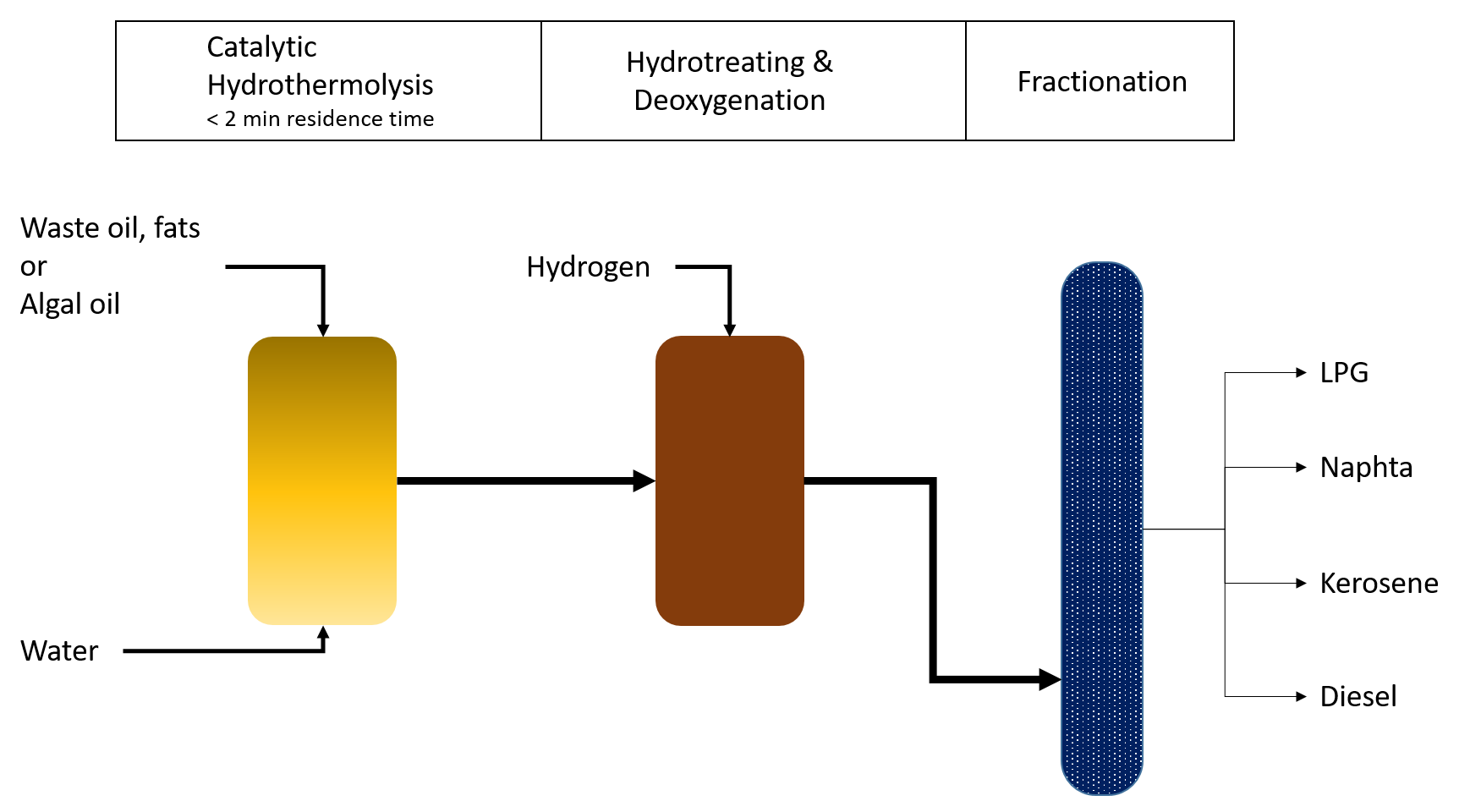
BIC process_EN by Anne Rödl, translated by HOOU (CC BY-SA 4.0)
Another option for biofuel production is co-refining of biobased oils with crude oil in conventional refineries. The composition and molecular structure of the two types of oil differ. For example, the carbon content of vegetable oil is lower than that of crude oil, but the oxygen content is higher. However, the exact composition depends on the feedstock. Process challenges, such as unwanted by-products or components like oxygen, make the process tricky.
Neuling, U. and Kaltschmitt, M., 2018. Biokerosene from Vegetable Oils – Technologies and Processes. In: M. Kaltschmitt and U. Neuling, (Hrsg.) Biokerosene. Status and Prospects. Berlin, Heidelberg: Springer, S. 475–496
