3. Plant-based pharmaceuticals
| Website: | Hamburg Open Online University |
| Kurs: | Process engineering for the bioeconomy |
| Buch: | 3. Plant-based pharmaceuticals |
| Gedruckt von: | Gast |
| Datum: | Freitag, 30. Januar 2026, 00:40 |
Beschreibung
In this book you will learn about the different groups of active ingredients and procedures.
3. Plant-based pharmaceuticals
As plants can neither run away from enemies nor from changing environmental conditions, they have developed other strategies to protect themselves. Secondary plant substances help to protect themselves from predators, keep fungi or bacteria away, attract pollinators or even keep conspecifics away. Secondary plant substances are very specific to certain species or genera and are not found uniformly in all plants. They are usually complex mixtures of substances, which then develop the respective effect. For the most part, it is not just one substance that is effective, but the entire cocktail. A distinction can be made between ingredients that determine efficacy and substances that contribute to efficacy. The latter can, for example, improve absorption in the body.
In most cases, people use the complete mixture of substances extracted from the plant in various ways (phytopharmaceuticals). In some cases, individual pure substances are also isolated and processed like chemically synthesized drugs, such as menthol (from field mint), morphine (from the opium poppy) or digoxin (from the woolly foxglove).

For thousands of years, humans have been using a variety of plant-based active ingredients to treat illnesses and ailments. The discovery of the medicinal effects of certain plant substances has had a decisive influence on today's understanding of medicine. Up to 70 % of medicinal substances are derived from natural substances. Today, some important active pharmaceutical ingredients are artificially synthesized based on nature, such as acetylsalicylic acid, known as the painkiller aspirin, which occurs naturally in willow bark.


3.1 Groups of active ingredients
Terpenes
Terpenes or terpenoids can have very different structures, but are very characteristic of the respective plant. The term terpene covers a wide range of substances, but they are all based on the molecule isoprene (C5H8), which is converted into the activated form isopentenyl diphosphate by incorporating phosphate.
The above-mentioned taxol used to combat cancer from the Pacific yew belongs to the terpene group, as does rubber, which has important technical and economic significance.
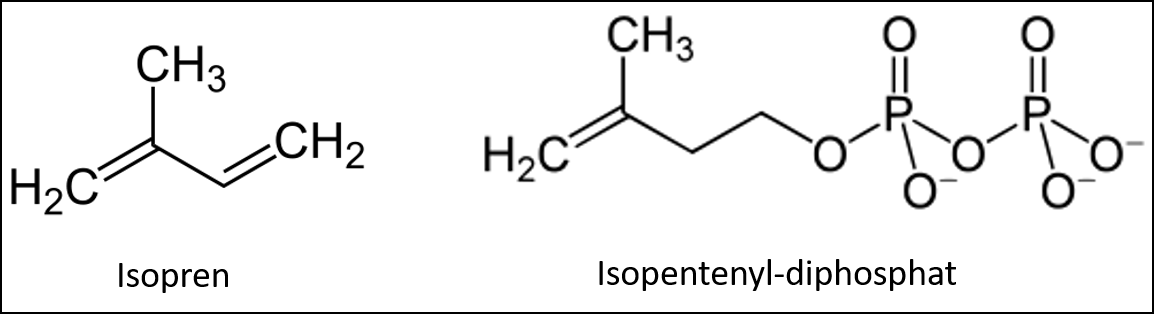
Terpenes are basically hydrocarbons with characteristic functional groups and chemically belong to the lipids.
Terpenes are divided into hemi- (C5), mono- (C10), sesqui- (C15), diterpenes (C20), etc. based on the C-atoms they contain. If there are more than 40 C atoms, they are referred to as polyterpenes.
| From | Substance | Occurence | Effect/Use |
| Monoterpene | Pines | Conifers | Turpentine / rosin |
| Sesquiterpene | Curcumene | Tumeric | Spice, good for digestive track |
| Diterpene | Taxol | Pacific yew | Cancer medication |
| Triterpene | Limonoide | Citrus fruits | Prevention of cancer, potentially lowering cholesterol |
| Tetraterpene | Carotinoids | Carrots | Strengthening immune system and sight |
| Polyterpene | cis-Polyisoprene | Rubber tree | Rubber |
Plants synthesize terpenes for a wide variety of applications:
- to repel predators (antimicrobial)
- to attract pollinators (odorants and flavorings)
- as signalling substances, growth regulators (phytohormones)
It is also interesting to note that some insects absorb plant terpenes and synthesize pheromones (signaling substances) from them, which they can use to attract or warn conspecifics.
Essential oils
Essential oils mostly consist of mono- and sesquiterpenes and form their own diverse subgroup of terpenes with a wide variety of medicinal effects. Essential oils have a characteristic, strong smell and taste, are oily but still very volatile. They are not readily soluble in water, forming a film on the water surface, but dissolve in lipophilic solvents (benzene, ethanol, fatty oils). Under the influence of light or oxygen, the essential oils change and lose their quality and taste.
Essential oils play a role in all smelling or fragrant parts of plants. They attract insects or protect the plant from predators, microorganisms or fungi.
Volatile essential oils are obtained by steam distillation. Less volatile essential oils are extracted by pressing, supercritical CO₂ or solvents. Essential oils can be used in a variety of ways as ointments, teas, rinses, tinctures or herbal seasonings. Depending on their origin, they promote circulation, stimulate appetite and digestion, have an antiseptic, calming, expectorant or anti-inflammatory effect.
| Essential oil | Effect |
| Anis oil | Simulates appetite and digestion |
| Citrus oils | Mood-lifting, stimulating |
| Eucalyptus oil | Antiseptic, antimicrobial |
| Camomile oil | Anit-inflammatory |
| Camphor oil | Stimulates blood ciculation |
| Lavender oil | Calming |
| Mentol | Disinfectant, cooling |
| Peppermint oil | Simulates appetite and digestion |
| Rosemary oil | Stimulates blood ciculation |
| Sage oil | Antiseptic |
| Thyme oil | Stimulates expectoration |
Phenolic compounds
The group of phenols or phenylpropanoids are very diverse and serve the plant both as a defense against predators or pathogens (tannins, phytoalexins), as well as to attract pollinators or seed dispersers (dyes) and to build supporting structures (lignin). The starting point for the synthesis of plant phenols is the amino acid phenylalanine.
The most important substance groups and their occurrence are described in more detail below.
Flavonoids (colorants)
The term flavonoids is derived from the Latin word for yellow “flavus”. Many colorants extracted from plants in the past (e.g. from the dyer's woad) were yellow. But other flower dyes (reddish, violet, bluish) also belong to the flavonoids. They also provide the plant with UV protection. Medicinally effective flavonoids are usually bound to sugars and are then referred to as glycosides. Many fruits and vegetables contain flavonoids. They are said to have a health-promoting, antioxidant, anti-carcinogenic and radical-scavenging effect.
Flavonoids are found in
- local fruit varieties (e.g. apples, pears, berries, cherries)
- onions, eggplants
- Tea, cocoa and red wine
Red vine leaves, for example, contain quercitrin and rutin, which have an anti-inflammatory and vascular-sealing effect and are therefore used to treat swollen legs.
Phytoalexins
Phytoalexins can also be classified as flavonoids, but can also be formed from other substance classes and should therefore be presented separately. In contrast to the dyes, they fulfill a completely opposite task. They serve as a defense against pathogenic fungi or bacteria. It is interesting to note that these substances are only produced after the plant has been infected and do not occur in healthy plants. Phytoalexins are designed to inhibit the spread or multiplication of harmful invaders. The phytoalexins can be detected in the infected plant parts after just 24 hours. Research into this is still relatively new.
Tannins (tannins)
Tannins are polyphenols and have both technical and medicinal significance. Tannins precipitate proteins and are therefore used in the production of leather from animal hides. Nowadays, however, synthetic tanning agents are mostly used in industry. Plants produce tannins to keep predators away. They are found in wood and bark (e.g. oak), but also in leaves and sometimes in fruit. Tannins have an antibacterial or antiviral effect and therefore have preservative properties. As they react with protein molecules, tannins have an astringent effect, i.e. they contract, which is used to treat diarrhea, increased sweating or inflammation.

Lignin
Lignin also belongs to the group of complex phenolic compounds (phenylpropanoids). They lead to the lignification of the plant cell wall and thus serve to increase the compressive strength. The technical use of lignins is currently still being researched, as lignins usually have to be laboriously separated from the wood for paper production or biofuel production, for example, and are then mainly used for energy.
The article by Rinaldi et al. (2016) offers a deeper insight into the state of research on better utilization of lignin:
https://doi.org/10.1002/ange.201510351
Alkaloids
Alkaloids are based on nitrogen-containing compounds. Most of them have a very strong effect on the central nervous system and are often toxic. Prominent representatives of this very extensive group of substances are caffeine, nicotine, cocaine, morphine, quinine or atropine (in belladonna). 12,000 alkaloids have been researched. The plant forms alkaloids in the amino acid metabolism and thus protects itself from predators. Each plant family produces a typical substance and only forms it in certain life stages or parts of the plant. This is why plants such as potatoes, tomatoes and peppers, which often belong to the nightshade family and produce alkaloids, are nevertheless important edible plants. In the consumed parts of these plants, the alkaloid is broken down with increasing maturity or disappears at the latest through preparation (peeling, cooking). In some cases, people deliberately accept the slightly toxic but stimulating effect. Tobacco, for example, also belongs to the nightshade family.
| Substance | Occurence | Effect/Use |
| Morphin | Opium poppy (Papaver somniferum) | Painkiller |
| Colchicine | Autumn crocus (Colchicum autumnale) | gout medicine |
| Atropine | Belladonna (Atropa belladonna), datura (Datura stramonium); angel's trumpet (Brugmansia) | ophthalmology (dilated pupils), antidote to pesticides or neurotoxins, anesthesia |
| Theobromine | Aca tree (Theobroma), cola nut (Cola acuminata) | stimulating, mood-enhancing |
| Capsaicin | Paprika/chilli (Capsicum) | antibiotic, increases blood circulation, against muscle pain and tension, “pepper spray” |
| Quinine | Yellow cinchona (Cinchona officialis) | malaria treatment |
| Ergotamine | Mother horn mushroom (Claviceps purpurea) | migraine and severe headaches (production of the drug LSD) |
| Strychnine | Nux vomica (Strychnos nuxvomica) | highly toxic, stimulating effect, causes cramps in high doses, used for homeopathic medicines, rat poison, doping) |
3.2 Process for the extraction of active plant ingredients
Active plant ingredients that are contained in different parts of the plant are mixtures of solids (plant parts), sometimes also mixtures of solids and liquids (e.g. soft fruits). There are suitable separation processes for each of these mixtures.
If you would like to get an initial general overview of physical-chemical separation processes, you will find a good overview here:
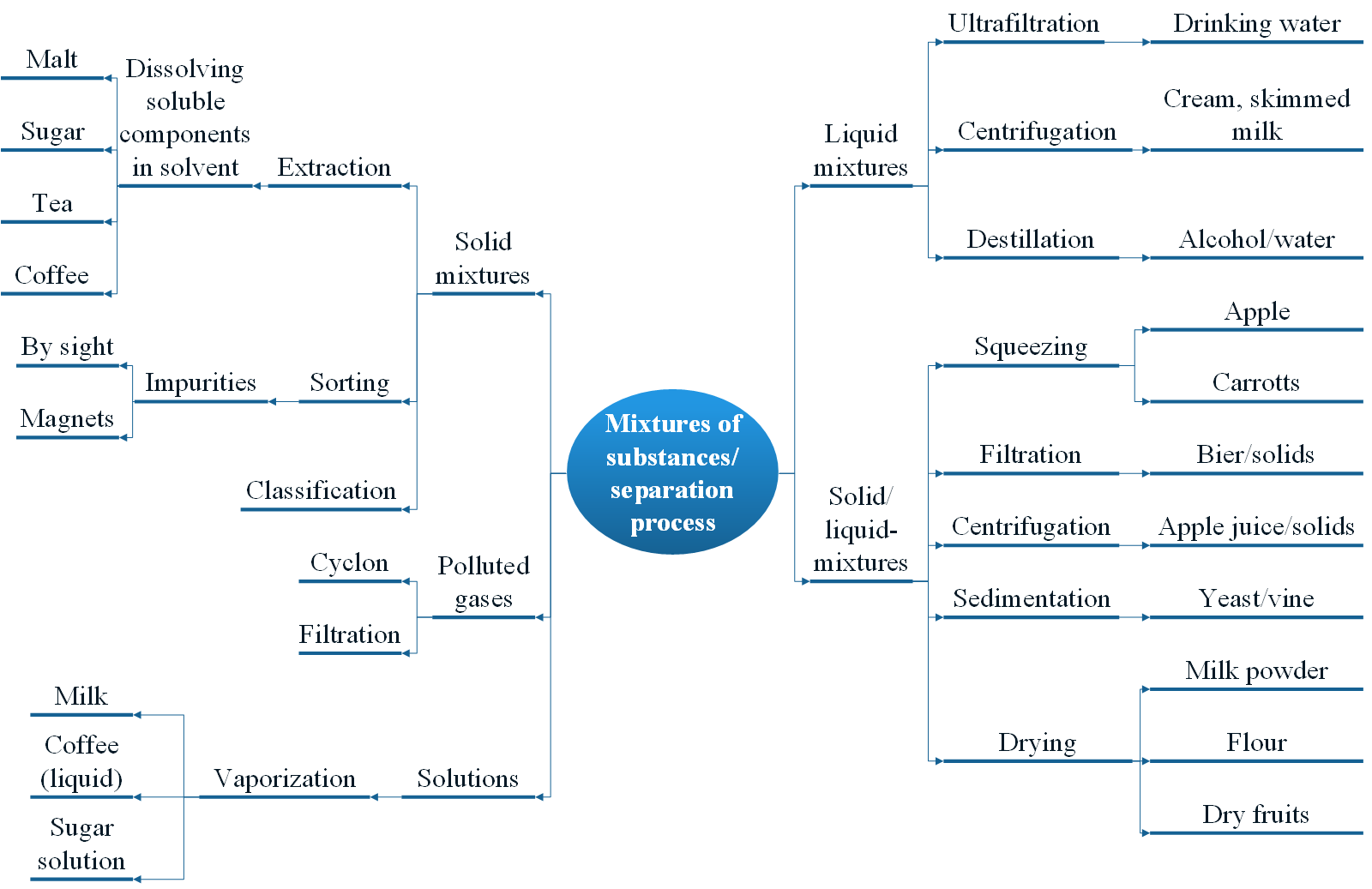
Active plant ingredients are usually obtained by extraction processes. Pressing is suitable for solid-liquid mixtures such as fruits containing oil or juice. For the most part, the entire mixture of substances is extracted from the plants.
If you would like to find out more about the mechanisms of the various separation processes, you can watch this video.
Extraction
Separation of a component of a mixture of solids using a solvent. It is based on different solubilities of the substances to be separated.
It is often a multi-stage extraction and purification process. Unwanted ingredients are removed and the desired active ingredients are enriched.
Suitable solvents are, depending on the properties of the substance to be extracted:
- Water
- Alcohol
- Oils
- Subercritical carbondioxide
General process steps
In the first step, it is usually advisable to crush the extraction material in order to break down the plant cells and increase the surface area on which the solvent can act. This is followed by intensive mixing of the substance mixture and solvent. The substance to be dissolved accumulates in the solvent. After several extraction passes, two different phases are obtained. The extract consists of the solvent mixed with the extracted substance and a residue called raffinate.
If both phases have a large difference in density, they can be separated from each other using a filter or separating funnel, for example. If they are homogeneously mixed, the solvent and extract can also be separated again by distillation or rectification (several distillations in succession). Both substances should have a significantly different boiling point.
Several dissolution and separation steps are often required to obtain pure substances. The following is a brief description of an extraction process commonly used in the pharmaceutical-technical field.
Overview of extraction processes
Percolation
Percolation is used when the solvent can flow easily through the extraction material.
A warm solvent is passed through the plant parts. The brewing of coffee in a coffee filter is usually cited here as an illustrative example. Several percolators connected in series are called repercolation.
Maceration
Also called cold extract. The plant parts are placed in the solvent (water, oil, alcohol) and left to infuse for some time. The soluble components pass into the solvent (macerate).
If the process is carried out at higher temperatures, it is called digestion.
Digestion
The shredded plant material is mixed with the solvent while heat is applied. Over time, the non-soluble components settle to the bottom. The solids and the extract are separated by decanting or filtering.
Here, too, there is an illustrative example from the world of coffee preparation. Brewing coffee using a French press pot corresponds to the principle of digestion. The hot water (solvent) is completely mixed with the extraction material (coffee) and only separated again after a while. The solvent is thus concentrated further and further with the extract substances. However, this also means that the extraction process becomes slower and slower as the concentration of the extract in the solution increases, meaning that completely different substances are dissolved from the extraction material than if the solvent is always fresh. The process must therefore be stopped by separating the extract from the solvent. Decanters (solid bowl screw centrifuge) can be used for this purpose. The suspension rotates in the horizontal bowl, as in a centrifuge. The deposited solids are continuously conveyed outwards by a screw.
In general, centrifuging is a suitable separation process for solid-liquid mixtures and you can take a closer look at the principle of a decanter here: ProjektWiki on decanter
Technically more advanced processes allow, for example, better mixing of the solids and the solvent using agitators, such as in turbo or vortex extraction. The use of ultrasound also causes the extraction mixture to vibrate and thus ensures more intensive mixing during extraction.
The desired components can also be extracted by boiling, provided they are heat-resistant. Plant dyes, for example, can be obtained in this way.
An experiment on this is described here: Abpflanz.PDF
Destillation
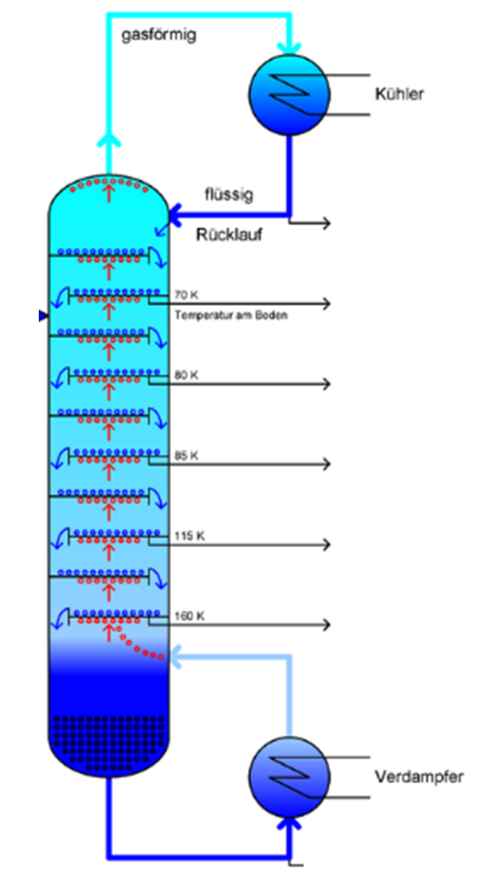
Essential oils or hydrolates (plant water) are often obtained by distillation. Water is mixed with the plant parts and heated. The vapor is collected, cooled in a condenser and thus becomes liquid again. This liquid condensate contains the dissolved plant ingredients and is collected. By repeating the process several times, the concentration of the extracted substances in the distillate can be increased. Large-scale plants use so-called rectification columns, in which the distillation process is repeated continuously as the steam rises and the condensate flows downwards.
Soxhlet extraction
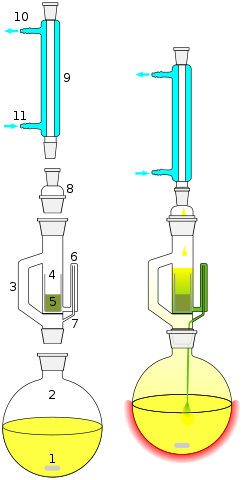
A laboratory process that enables and refines the extraction of soluble substances from solids through a combination of distillation and percolation is the Soxhlet process using a Soxhlet apparatus.
- The solid is filled into the attachment (5)
- Solvent is heated underneath (1)
- Steam rises in the steam pipe (3) condenses (9) and drips through the attachment into the solids
- When a certain filling level is reached, the solvent, which in the meantime has dissolved some of the solid, overflows into the thin tube (6) and back into the flask.
- Gradually, more and more of the dissolved substance accumulates in the flask until finally all the solvent has evaporated
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Beim Abspielen wird eine Verbindung zu den Servern von YouTube hergestellt und der Anbieter kann Cookies einsetzen, die Hinweise über dein Nutzungsverhalten sammeln.
Column chromatography
A tall glass column is used here, which is filled with a solid or powdery packing material. This packing material is called the stationary phase and usually consists of silica gels, aluminum oxide or calcium carbonate, sometimes also of porous, synthetic polymers. It can fill the entire column or only cover the inner surfaces. There is a tap at the bottom of the column. The plant material dissolved in a suitable solvent (acetone, hexane, ethanol, methanol, etc.) is applied to the column. This mixture is called the mobile phase. In order to transport it through the column, a so-called running agent is continuously dripped on from above. Different polarities of the substances in the mobile phase cause interactions with the stationary phase. These cause the mobile phase to separate, as the substances migrate through the solid at different speeds. They separate in zones and can be discharged individually.
Column chromatography can also be used as an analytical instrument by determining and measuring the individual components of the mixture after separation.
On a commercial scale, the process is accelerated by forcing the mobile phase through the stationary phase under pressure.
This video in the Lecture2Go series from the University of Hamburg explains the column chromatography process in detail:
Video Lecture2Go: Naturstoffe - SäulenchromatographieHigh-pressure gas extraction
The extraction of solids can also be carried out with a supercritical fluid instead of liquid organic solvents. This is particularly helpful in the food and pharmaceutical sectors, as there is no need to work with solvents that are sometimes toxic or harmful to health. The process uses so-called supercritical carbon dioxide (CO₂), which assumes a state between gas and liquid under high pressure and high temperature. With the help of a pump or a compressor, the heated CO₂ is passed through the plant material and dissolves the desired substances. The extract is separated in a separator by expanding the CO₂. The process is particularly suitable for low-volatility but thermally unstable substances.