2.1 Hydrogen and different Hydrogen Production models
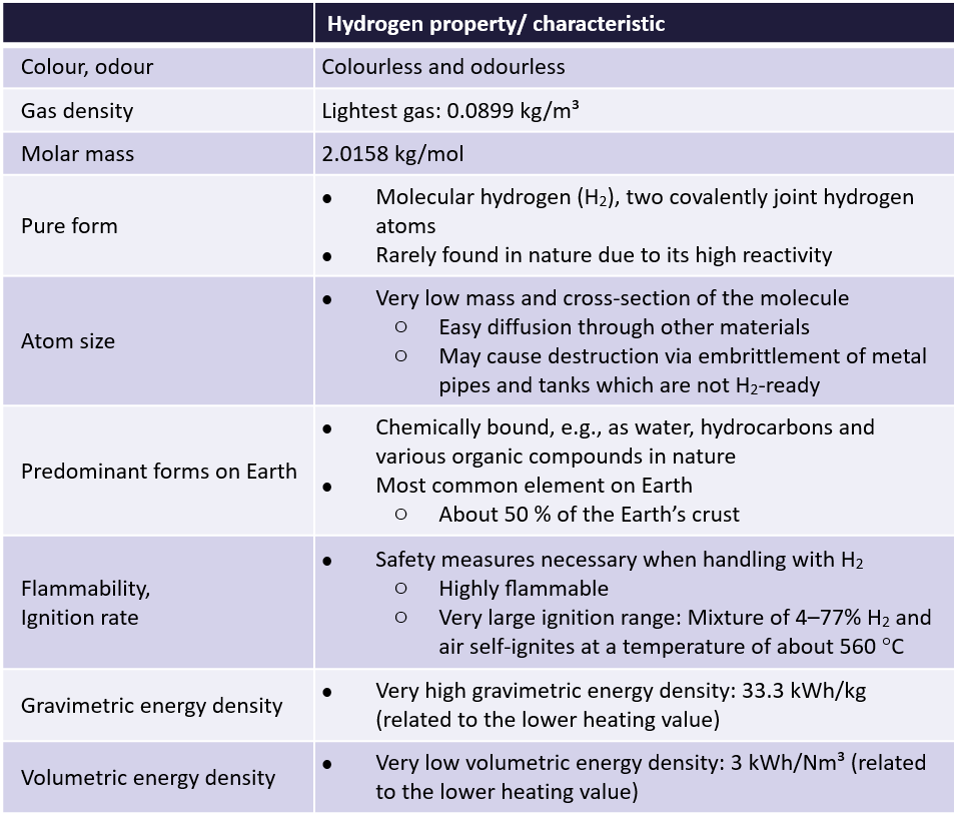
Properties and characteristics
Hydrogen is the first element of the periodic table of elements and thus has the lowest atomic mass of all elements. Therefore, when planning on using hydrogen as an energy carrier, it has to be taken into account, that the handling of hydrogen is different compared to other gases or substances with respect to its properties and very own characteristics. Some of those are stated as a short overview in the table below.
The figure shows that liquid energy carries, such as diesel fuel or petrol (= gasoline), have a significantly larger volumetric energy density compared to hydrogen. Due to the logarithmic scaling of the y-axis, the difference between hydrogen and the other energy carriers in terms of volumetric energy density is actually even higher than it appears at first glance at the diagram. At ambient pressure, even the volumetric energy density of natural gas exceeds that of hydrogen by a factor of about 3. Thus, for hydrogen use either very large tank volumes are needed or an increase of hydrogen’s volumetric energy density is necessary via compression or even liquidisation. More details regarding these challenges will be provided in chapter 3. On the other hand, the figure also shows that hydrogen clearly outperforms other energy carriers in terms of gravimetric energy density.